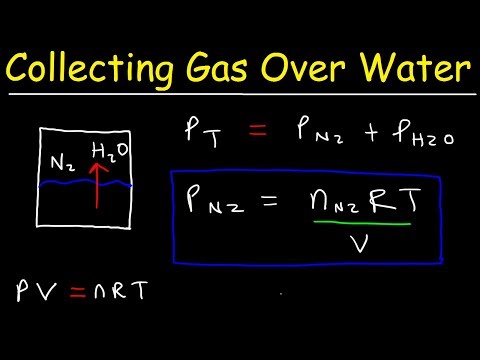
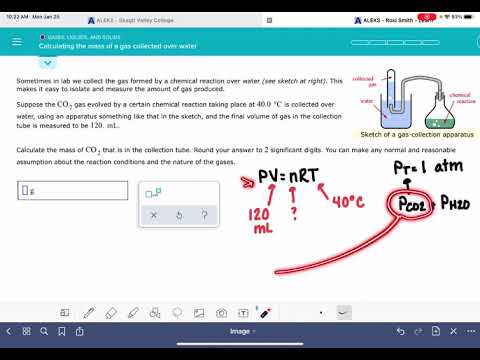
When a reaction produces a gas that is collected above water, the trapped gas is a mixture of the gas produced by the reaction and water vapor. Water evaporates and there is always gaseous water above a sample of liquid water. As a gas is collected over water, it becomes saturated with water vapor and the total pressure of the mixture equals the partial pressure of the gas plus the partial pressure of the water vapor. The pressure of the pure gas is therefore equal to the total pressure minus the pressure of the water vapor—this is referred to as the "dry" gas pressure, that is, the pressure of the gas only, without water vapor. The ideal gas law can be used to derive a number of convenient equations relating directly measured quantities to properties of interest for gaseous substances and mixtures.
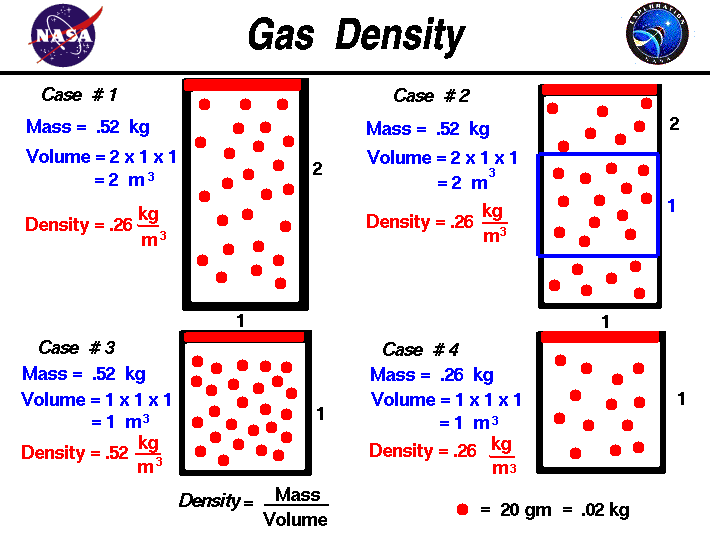
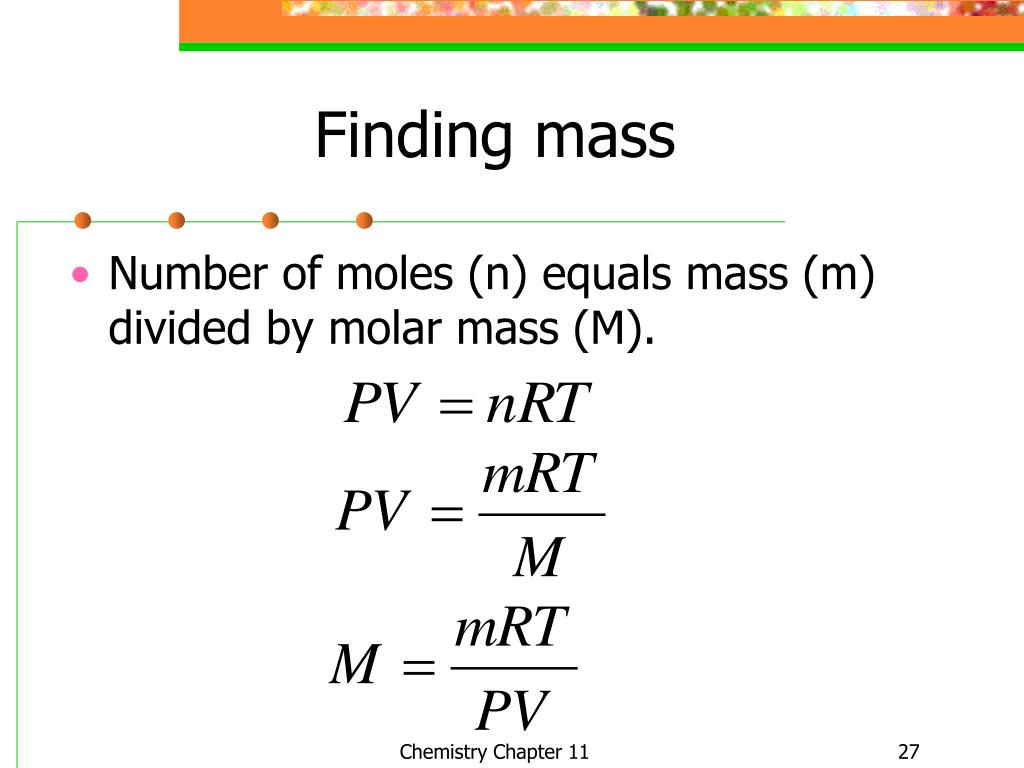
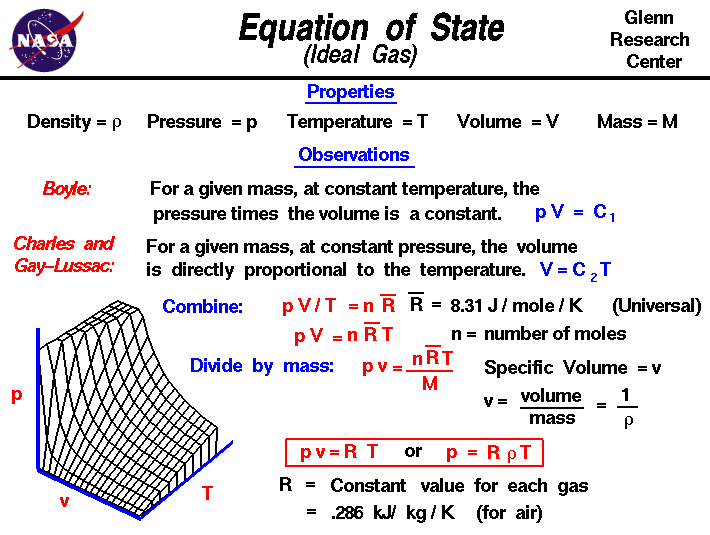
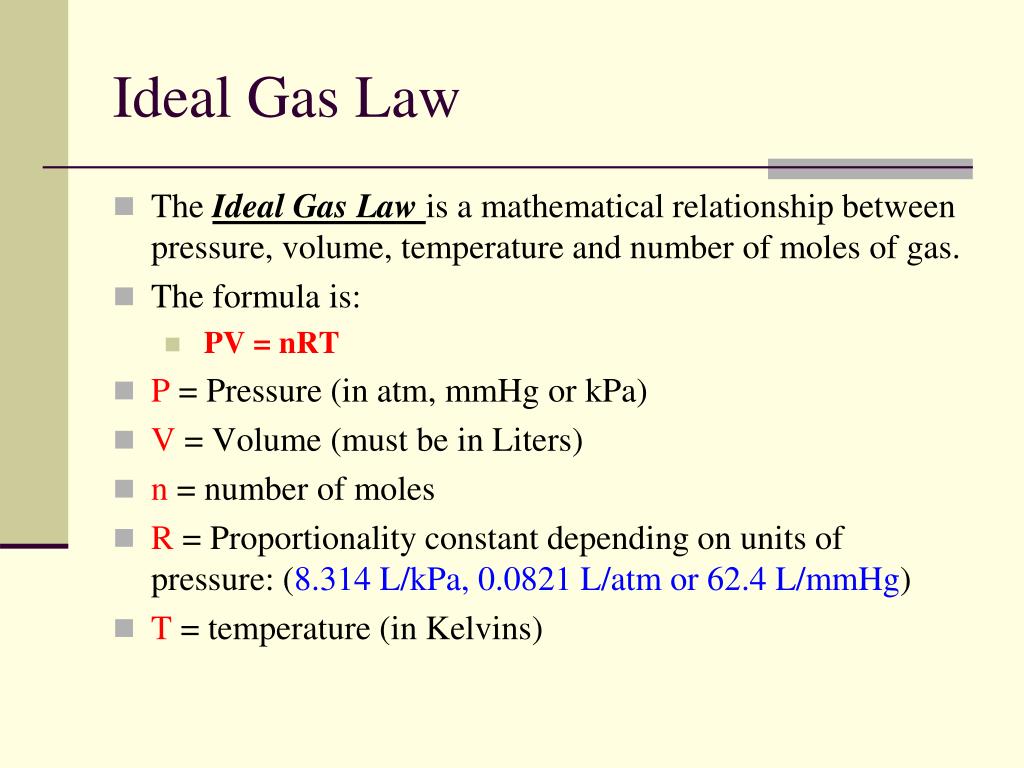
Appropriate rearrangement of the ideal gas equation may be made to permit the calculation of gas densities and molar masses. Dalton's law of partial pressures may be used to relate measured gas pressures for gaseous mixtures to their compositions. Avogadro's law may be used in stoichiometric computations for chemical reactions involving gaseous reactants or products.
Apply an approximate correction factor using Dalton's Law, which states that the sum of the individual partial pressures of the gases in a mixture (P1…Pn) is equal to the total pressure . Estimate the amount of water vapor in the hydrogen at other temperatures by using the partial pressure of water vapor at the temperature in question. A sample of hydrogen gas was collected over water at 60°C. If the total volume of gas collected was 80.9 mL and the atmospheric pressure was 1.03 bar, what mass of hydrogen gas was collected above the water? Dalton's law of partial pressure has a practical application in calculating volumes of gases collected over water.
This is a common way of collecting a not very soluble gas, for example, oxygen that has been prepared in the laboratory. The oxygen gas collected in this was is not pure because water vapour is also present in the same space. The total gas pressure is equal to the sum of the pressure exerted by the oxygen gas and the water vapour, which can be measured with a barometer. There is a growing research interest in the development of portable systems which can deliver hydrogen on-demand to proton exchange membrane hydrogen fuel cells. Researchers seeking to develop such systems require a method of measuring the generated hydrogen.
Herein, we describe a simple, low-cost, and robust method to measure the hydrogen generated from the reaction of solids with aqueous solutions. The reactions are conducted in a conventional one-necked round-bottomed flask placed in a temperature controlled water bath. The hydrogen generated from the reaction in the flask is channeled through tubing into a water-filled inverted measuring cylinder. The water displaced from the measuring cylinder by the incoming gas is diverted into a beaker on a balance. The balance is connected to a computer, and the change in the mass reading of the balance over time is recorded using data collection and spreadsheet software programs. The data can then be approximately corrected for water vapor using the method described herein, and parameters such as the total hydrogen yield, the hydrogen generation rate, and the induction period can also be deduced.
The size of the measuring cylinder and the resolution of the balance can be changed to adapt the setup to different hydrogen volumes and flow rates. The laboratory methods specified for this procedure are based on American Standards and Testing Materials , US Environmental Protection Agency , and Gas Processor Association methods. These laboratory methods measure the volume and composition of gases that flash from the liquids, including a Gas-Oil or Gas-Water Ratio, as well as the molecular weight and weight percent of the gaseous compounds.
Included are procedures for measuring the bubble point pressure and conducting a laboratory flash analysis. The laboratory results are used with the crude oil or condensate or produced water throughput to calculate the mass of emissions that are flashed from the liquids per year. Step 6 _ The molar volume of all gasses at STP is supposed to be the same.
It is known and you would not need to determine it experimentally. If as a lab exercise you are supposed to determine it from the data of this experiment, you would take the volume, and number of moles and calculate the volume per mol. Then, using the temperature and partial pressure of hydrogen measured/calculated, correct that volume to STP conditions.
It should come close to the known molar volume of all gasses at STP. If not, the difference is experimental error, calculation error, or some flaw in the reasoning (maybe even the assuming that the gas collected is also at 23.2 degrees). In the tube, a mix of hydrogen gas and vapor water will collect.
For starters, I would assume that the temperature of the gas and everything else has equalized, and the entire lab is at 23.2 degrees. If that was not the intention of the author of the problem, we should find out when numbers do not agree. You had a long tube filled with water inverted over a container with water. The tube ended with water to a level 460.0 mm higher than the level of the water in the outside container.
The water level difference indicates a pressure difference. Maybe you can find a conversion factor between mm water and torr. Otherwise, you would know that a 13.6 mm column of water exerts the same pressure than a 1.0 mm column of mercury because the density of mercury is 13.6 times the density of water. You will begin by reacting a known mass of magnesium metal with an excess of hydrochloric acid. The hydrogen gas produced will be collected by displacing water in a gas collection tube. Whenever a gas is collected over water, the result is a mixture of the collected gas and water vapor.
Eventually, these individual laws were combined into a single equation—the ideal gas law—that relates gas quantities for gases and is quite accurate for low pressures and moderate temperatures. We will consider the key developments in individual relationships , then put them together in the ideal gas law. The partial pressure of water vapour above liquid water is called its vapour pressure, which depends on the temperature. At room temperature the vapour pressure of water is only about 20 Torr. The following shows that we can use Dalton's law to calculate the amount of gas collected over water.
Many chemistry and physics experiments involve collecting the gas produced by a chemical reaction and measuring its volume. Water displacement represents one of the easier methods to accomplish this task. The technique typically involves filling a glass column open on one end with water and then inverting the column and submerging the open end in a bowl of water. Columns built specifically for this purpose are called eudiometer tubes. The determined volume of a gas becomes useful only if the pressure of the gas is also known.
This requires equilibration of the pressure inside the tube with atmospheric pressure. Oxygen gas is collected over water at a temperaturee of 10 degrees C and a pressure 1.2 atm. The volume of gas plus water vapor collected is 293ml.
Q. The hydrogen gas formed in a chemical reaction is collected over water at 30.0 °C at a total pressure of 732 mmHg. This procedure is used to determine the bubble point pressure at sample collection temperature of a pressurized hydrocarbon liquid prior to conducting a flash or any compositional analysis. These results determine the integrity of the sample and provide a means of verifying the sampling conditions reported on Form 1. When heating is required, safety precautions must be taken due to thermal expansion within a pressurized cylinder. This procedure is performed with the use of a Double Valve cylinder and is not applicable for Floating-Piston cylinders.
Samples gathered with the use of a Floating-Piston cylinder must be transferred to a Double Valve cylinder using a water displacement method prior to conducting this procedure. This procedure is used for determining the annual flash emission rate from tanks used to separate, store, or hold crude oil, condensate, or produced water. The laboratory methods required to conduct this procedure are used to measure methane and other gaseous compounds. Consider gas which has been collected over water to be saturated with water vapor. During the collection process, the water level in the measuring cylinder adjusts to maintain the internal pressure in the measuring cylinder at atmospheric pressure. Rather than being manually recorded, the data is logged in a spreadsheet using a data collection software package which allows the balance to send data to the computer.
A sample of hydrogen gas is collected over water at 25 c. Volume of sample pressure of sample vapor pressure of h;0 (25°c) 82. A gas produced in a chemical reaction can be collected by water displacement. Because the gas is collected over water, it is not pure but is mixed with vapor from the evaporation of the water. Dalton's law can be used to calculate the amount of the desired gas by subtracting the contribution of the water vapor.
The ideal gas law describes the relationship between the pressure, volume, temperature, and number of moles of an ideal gas. Discover the ideal gas law equation and explore sample problems and solutions involving the ideal gas law. We have previously measured quantities of reactants and products using masses for solids and volumes in conjunction with the molarity for solutions; now we can also use gas volumes to indicate quantities. If we know the volume, pressure, and temperature of a gas, we can use the ideal gas equation to calculate how many moles of the gas are present. If we know how many moles of a gas are involved, we can calculate the volume of a gas at any temperature and pressure. This procedure is conducted by collecting samples of crude oil or condensate and produced water upstream of a separator or tank where flashing may occur.
Samples must be collected under pressure and according to the methods specified in this procedure. If a pressure separator is not available for collecting samples, sampling shall be conducted using a portable pressurized separator. A sample of hydrogen gas is collected over water at 25 C.
Volume of Sample Pressure of Sample Vapor Pressure of H;0 (25°C) 82.33mL 745mmHg 23.8mmHg Determine the mass and root mean square velocity of the hydrogen gas collected. We can calculate the number of moles using the ideal gas law once we know the partial pressure, the volume and the temperature. The thin skin of our atmosphere keeps the earth from being an ice planet and makes it habitable. In fact, this is due to less than 0.5% of the air molecules.
Of the energy from the sun that reaches the earth, almost \frac[/latex] is reflected back into space, with the rest absorbed by the atmosphere and the surface of the earth. Some of the energy that the earth absorbs is re-emitted as infrared radiation, a portion of which passes back out through the atmosphere into space. However, most of this IR radiation is absorbed by certain substances in the atmosphere, known as greenhouse gases, which re-emit this energy in all directions, trapping some of the heat. This maintains favorable living conditions—without atmosphere, the average global average temperature of 14 °C (57 °F) would be about –19 °C (–2 °F). The major greenhouse gases are water vapor, carbon dioxide, methane, and ozone. Since the Industrial Revolution, human activity has been increasing the concentrations of GHGs, which have changed the energy balance and are significantly altering the earth's climate .
6 Nitrogen gas is collected over water in a measuring cylinder, the level of water being the same inside and outside the cylinder. The volume of nitrogen collected was 120 mL, the temperature was read as 21 oC. Calculate the volume of dry nitrogen obtained at 298 K and 101.3 kPa.
To investigate the reproducibility of the experimental set-up, varying masses of silicon were reacted with aqueous sodium hydroxide solutions to generate hydrogen. The average hydrogen generation curves are shown in Figure 1. Average total hydrogen yields, hydrogen generation rates, and induction periods for each mass of silicon were also calculated and are plotted with error bars representing one standard deviation in Figures 2, 3, and 4, respectively. There was very little deviation in the total hydrogen yields and hydrogen generation rates between reactions, and a greater level of deviation in the induction periods. Oxygen gas generated in the decomposition of potassium chloride and collected over water at 20oC. The volume of the gas collected at atmospheric press 755 Torr is 370mL.
The pressure of the water vapour at 20oC is 17.5 Torr. A gas is collected over water at 30.0 degrees celsius. Water vapor pressure at twenty degrees Celsius is 27.5 mm Hg. The properties of N2 will deviate more from ideality at -100oC than at 100oC. Van der Waal's equation corrects for the non-ideality of real gases. Molecules of CH4 at high pressures and low temperatures have no attractive forces between each other.
Molecules of an ideal gas are assumed to have no significant volume. By automatically logging the data in a spreadsheet, this method offers a significant improvement in accuracy and temporal resolution with respect to water displacement methods which rely on recording the volume of gas evolved manually. 1.00g of air consists of approximately 0.76g of nitrogen and 0.24g of oxygen. Calculate the partial pressures and the total pressure when this sample occupies a 1.00L vessel at 20oC. So, for your question water vapor and the gas occupy the same volume. What is different is the partial pressure and the number of moles of each one.
The important thing to remember about gases that are being collected over water is that the total pressure measured includes the vapor pressure of water at that given temperature. In order to solve a problem, it is necessary to know the vapor pressure of water at the temperature of the reaction . The sample problem illustrates the use of Dalton's law when a gas is collected over water. The volume of gas collected and the gas laws can be used to calculate the number of moles of gas collected.
The partial pressure of a gas in a mixture is the pressure it would exert if it alone occupied the container. If we have gas A and B in a container, then partial pressures of A and B are expressed as PA, PB. Dalton then described the behaviour of gases mixture by his 'law of partial pressure'. Lower the eudiometer into the water-filled graduated cylinder prepared in step 1 until the water level inside the eudiometer tube is exactly even with the water level in the graduated cylinder. At this point, the pressure inside the eudiometer tube is equal to the pressure outside the tube, i.e., atmospheric pressure.
Now read the volume of the water level in the eudiometer tube. Because glassware manufacturers label the volume readings on eudiometer tubes from closed end to open end, this volume reading will reflect the volume of gas in the tube. An ideal gas is a hypothetical construct that may be used along with kinetic molecular theory to effectively explain the gas laws as will be described in a later module of this chapter.
Although all the calculations presented in this module assume ideal behavior, this assumption is only reasonable for gases under conditions of relatively low pressure and high temperature. In the final module of this chapter, a modified gas law will be introduced that accounts for the non-ideal behavior observed for many gases at relatively high pressures and low temperatures. Now you know how to calculate partial pressure using the ideal gas law. Let's move on to the last two forms of the partial pressure equation - both using Henry's law. Imagine that you need to do a lab experiment where hydrogen gas is generated.
In order to calculate the yield of gas, you have to know the pressure inside the tube where the gas is collected. All you need is the atmospheric pressure in the room. As the gas pushes out the water, it is pushing against the atmosphere, so the pressure inside is equal to the pressure outside. The pressure inside the bottle is partially from the gas being collected and partially from the water vapor that has escaped from the surface of the water in the jar. The water inside the jar will reach an equlibruim state where the number of molecules leaving the surface is the same as the number returning.